Physics of Biological Tissues
Collective cell behaviours guide embryonic development, tissue repair and can contribute to cancer metastasis. Although there is a growing recognition that cell behaviours can be governed and coordinated by collective tissue responses, the mechanisms that coordinate cell behaviours are remained to be unknown. In our research, we develop mathematical/physical models to answer two main questions to study the cell behaviours: (1) how can we define and control the material properties of tissues (collective response)?, and (2) do tissue material properties affect cellular functions and coordination thereof? To answer the former, we develop 2D and 3D models that incorporate the interplay between mechanical cues and biochemical signaling. To answer the latter, we apply these models to various developmental processes and develop verifiable predictions that can be tested in the experiments.
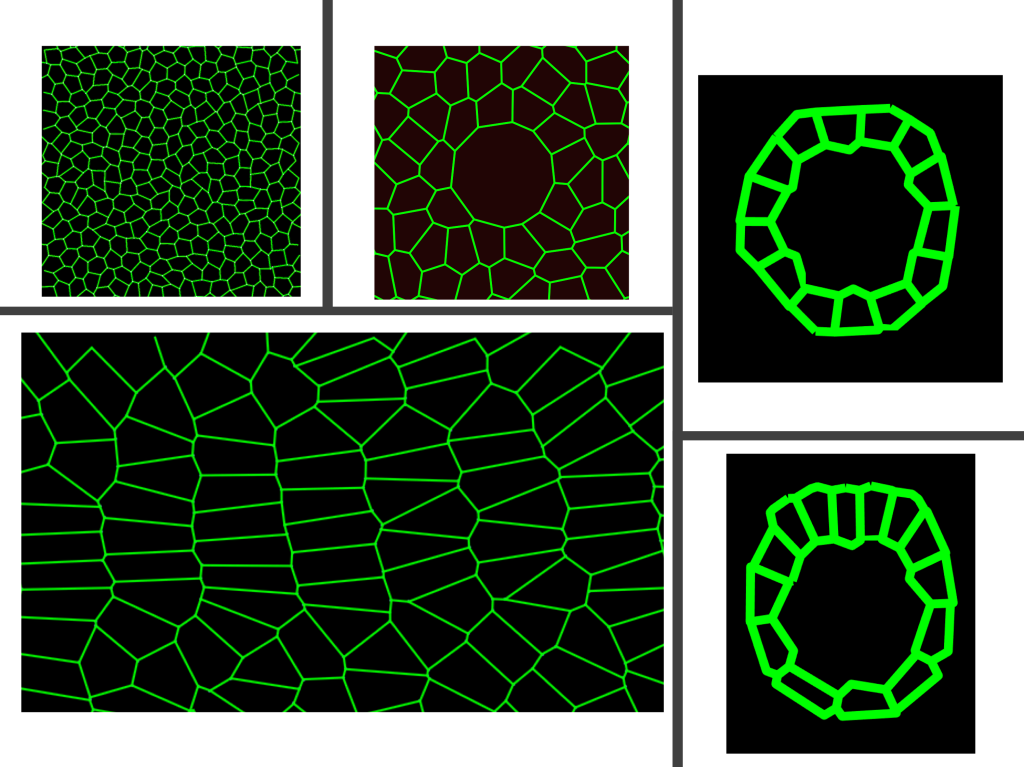
Impact of cellular rearrangement time on tissue mechanics. Vertex models offered a novel type of rigidity transition for biological tissues based on a preferred cell shape: the ratio of the average cell perimeter to the square root of the cell area that the cells would like to achieve. In these standard vertex models, the tissue act as a fluid-like above a critical cell shape and solid-like below the critical value. In the solid-like regime, cells are jammed and do not exchange neighbors (cellular rearrangement) whereas cells exchange neighbors without a cost of energy in the fluid-like regime. The cell exchanges proceed instantaneously in standard vertex model formulations. However, in vivo, cells rearrange in a finite time as a sequence of molecular processes must occur. We developed a model that incorporated the cellular rearrangement time into 2D vertex models. We showed that the celllular rearrangement time affects tissue material properties, stiffening the tissue. Our model suggested that the cellular rearrangement time regardless of the cell shape can control the tissue material properties (PLoS Comput. Biol. 2021).
Role of collective tissue mechanics on various developmental processes. In close collaborations with experimentalists, we develop verifiable mathematical models and predictions to study the role of tissue mechanics on various embryonic developmental processes. These projects involve embryonic organ development (Biophys J 2018, Nat Comm 2020, Cells & Development 2021), tissue boundary formation (Cells & Development 2021), fruit fly heart cell migration (Developmental Cell 2023), fruit fly body axis elongation (PNAS 2020), neuroblast cell ingression, and wound healing.
Physics of Viruses
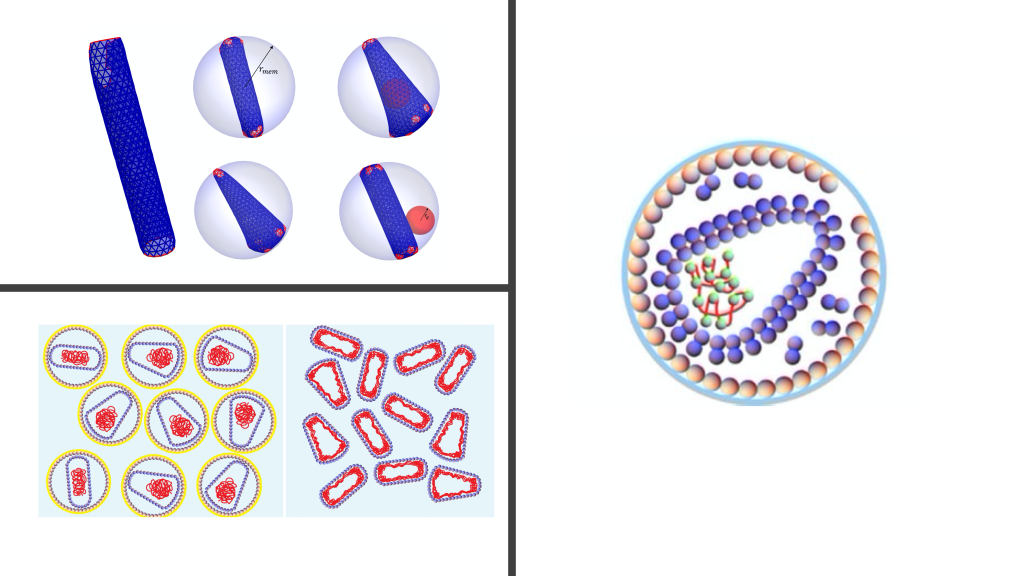
The simplest viruses are composed of a protein shell (capsid) that encapsulates the viral genome (RNA or DNA). RNA free in solution assumes a branched polymer structure due to its base-pairs. Our work on viruses focused on two main categories: (1) understanding the impact of RNA structure on spherical viruses using mean-field theories and (2) understanding the capsid formation of non-spherical viruses using coarse-grained simulations.
Modelling RNA of viruses. While the microscopic description of viral RNAs is not yet deciphered, the RNA density of most spherical viruses can be categorized into three main profiles. However, it is not known why there is a variation in the profile. Our work was the first to capture all different types of RNA profiles in a model (PRL 2017) where we studied the impact of base pairing on the conformations of RNA inside viral shells. We showed that a small change in the number of RNA base pairs or genome length can result in drastic changes in the profile of RNA.
Modelling HIV viral shell formation. HIV buds from infected cells as a non-infectious immature virus which is transformed into a mature infectious particle. Understanding the pathway that HIV takes to mature is crucial to develop antiviral therapies. We simulated HIV maturation using coarse-grained simulations to explore the underlying physical mechanisms and constraints on the formation of HIV shells, which are either cylindrical or conical. We showed that the frequency of formation of conical shells are higher when a part of the shell comes from a portion of an immature lattice (protein sheet of the immature virus) as opposed to coming from a completely detached immature lattice (detached proteins of the immature virus). This proposed a new kinetic pathway model for HIV formation which was supported by the experiments (Nat. Comm. 2016).
Full publication list
Copyright © 2021 Gonca Erdemci-Tandogan – All Rights Reserved.